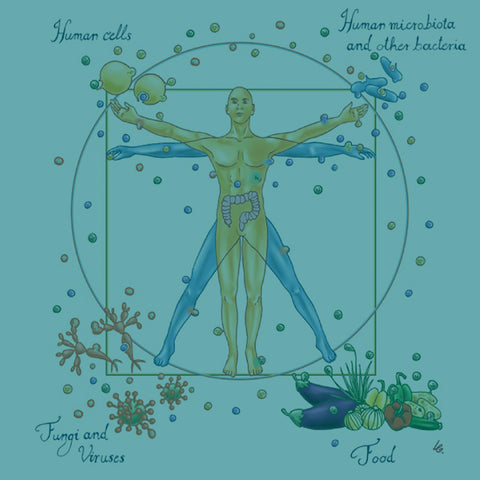
It may seem like an odd term, but "holobiont" essentially means an assemblage between a host and other species that form a discrete ecological unit.
Derived from the Greek term holos meaning whole or entire, the concept of a holobiont was initially defined by Dr. Lynn Margulis in her 1991 book, "Symbiosis as a Source of Evolutionary Innovation." As with most things, the definition has evolved considerably in the intervening years.
Each species present in the holobiont is an individual "biont," and the genomes of all bionts taken together are collectively called the hologenome or the "comprehensive gene system" of the holobiont [1,2]. The complex interactions between the host and its community of microbial organisms can result in benefit, damage, or an indifferent response [3].
This article will take a focused look at what a holobiont is, how they work, and why they are important in the context of health and disease states, with a special emphasis on the importance of gut microbiota.
Quick note: The holobiont concept is also the foundation of Health Optimization and Practice (HOMe/HOPe) Essential Certification for healthcare practitioners. HOMe/HOPe is the non-profit entity supported by Troscriptions. For more information, head to homehope.org!
Now, back to the meat (you) and the potatoes (the other bionts!)...
How Do Holobionts Work?
Holobionts are distinct from "superorganisms," and this concept is best illustrated using ants as an example. An ant colony is classed as a superorganism, with many individual ants making up the whole. Each one of these individual ants, along with its associated bacteria, fungi, and so on, is a holobiont.
The symbiosis between a host organism and its microorganisms is pivotal, as the host benefits from a biological and ecological perspective. The host is provided with vitamins, energy, inorganic or organic nutrients, as well as augmented defense mechanisms and evolutionary drive [4,5].
The components of a holobiont are the host (which tends to be a multicellular eukaryote such as a plant or human), the microbiome (i.e., bacteria, archaea), virome (all the viruses present in the holobiont), and fungi [6].
Are Humans Holobionts?
Humans are indeed holobionts – they comprise a host (i.e., us!) and trillions of microorganisms whose collective functioning keep the entire organism alive [7]. In fact, the number of microbial cells that colonize epithelial surfaces and live in a cloud around you is at least equivalent to, if not more than, the number of human cells [1,8].
Although the microbiome is the foremost bacterial population in the human body in terms of diversity [1,9,10] and will be addressed further below, the second most prominent is found in the oral cavity and its extensions (teeth, gums, tongue, cheek, lip, hard palate, etc.).
Humans and the Microbiome
The human gut microbiome is increasingly recognized for its integral role in health and disease [8,11]. The last 25 years or so have produced an avalanche of research studies investigating the impact of the microbiome on physiology and metabolism in multicellular organisms [12]. Astonishingly, metabolomics analysis has shown that as much as 10% of the metabolites in mammalian blood are derived from gut bacteria, a fact that illustrates the prominence of the microbiome [13]. In the HOMe/HOPe framework, we use organic acid testing and metabolomics to detect and correct various metabolites that correspond to bacterial dysbiosis, maldigestion, yeast overgrowth, and more.
When we’re born, our gastrointestinal or GI tract is sterile at first, but is then rapidly colonized by successive waves of microorganisms until a dense microbial population is stabilized at around the time of weaning [14]. This population consists of thousands of bacterial species, albeit from a small number of phyla (related organisms classified according to their fundamental characteristics) [15,16].
Of the 55 million or so bacterial divisions that exist on Earth, only two of these phyla, Bacteroidetes and Firmicutes, are predominant in the GI microbiota [17]. The coevolution of humans and infectious microorganisms is shown by the example of Helicobacter pylori, which was used to trace the migration of early humans from northern Africa [18].
The GI microorganisms have an essentially unlimited supply of nutrients and in return provide a range of benefits to immune function, such as vitamins, energy, nutrients, and enhancements to name a few [11,19]. Short chain fatty acids made by gut microbiota keep the lining of the gut healthy. Other bacteria make our serotonin and B-vitamins as well.
In the adult gut, the microbiota composition varies dramatically between individuals and is highly resistant to perturbation, but antibiotics, toxic exposures including plastics, and more can permanently perturb this ecosystem [20].
The Microbiome, Health, and Disease
Our understanding of the gut microbiota is of great relevance and importance with respect to complex human diseases such as obesity, coronary heart disease, diabetes, and inflammatory bowel disease [21,22].
We have known for over a century that changes in the diet can influence the type of metabolites produced by bacteria living in the healthy intestine, and that the composition of the intestinal microbiome itself is related to the composition of the diet as a whole [23,24].
Although the microbiome is resistant to perturbation, when this takes place (a phenomenon called dysbiosis), bacteria that are normally benign or even beneficial in the context of a healthy microbiome can promote chronic pathologies, such as atherosclerosis and obesity [25,26].
For example, consuming an obesity-inducing, Western-style diet that is typically low in fiber and high in calorie content from sugar, selects for a less diverse microbiota that efficiently extracts energy from these sources. When a mouse is "transplanted" with the microbiota of an obese individual, the ability to use energy from these sources is transferred with it, and the mouse becomes obese [21]. This is also seen in humans that undergo fecal microbiota transplants due to severe infections like clostridium difficile.
Summary
In short, we are not a population of individuals but more an individual made of populations... these populations being various types of cells that include your own, which are dwarfed in number compared to those coming from bacteria, fungus, and viruses. And not only these cells but also various components of our environment such as the foods we eat, the water we drink, and the toxins we are exposed to (i.e., the exposome).
The good news? We can measure using metabolomics. If you are a practitioner or if you are someone interested in learning more about your metabolome, check out homehope.org for more information!
References
[1] S.R. Bordenstein, K.R. Theis, Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes, PLoS Biol. 13 (2015) e1002226. https://doi.org/10.1371/journal.pbio.1002226.
[2] K.R. Theis, N.M. Dheilly, J.L. Klassen, R.M. Brucker, J.F. Baines, T.C.G. Bosch, J.F. Cryan, S.F. Gilbert, C.J. Goodnight, E.A. Lloyd, J. Sapp, P. Vandenkoornhuyse, I. Zilber-Rosenberg, E. Rosenberg, S.R. Bordenstein, Getting the Hologenome Concept Right: an Eco-Evolutionary Framework for Hosts and Their Microbiomes, MSystems. 1 (2016) e00028-16. https://doi.org/10.1128/mSystems.00028-16.
[3] A. Casadevall, L.-A. Pirofski, What is a host? Incorporating the microbiota into the damage-response framework, Infect Immun. 83 (2015) 2–7. https://doi.org/10.1128/IAI.02627-14.
[4] E. Rosenberg, I. Zilber-Rosenberg, Microbes Drive Evolution of Animals and Plants: the Hologenome Concept, MBio. 7 (2016) e01395-15. https://doi.org/10.1128/mBio.01395-15.
[5] K. Ugarelli, S. Chakrabarti, P. Laas, U. Stingl, The Seagrass Holobiont and Its Microbiome, Microorganisms. 5 (2017) 81. https://doi.org/10.3390/microorganisms5040081.
[6] P. Vandenkoornhuyse, A. Quaiser, M. Duhamel, A. Le Van, A. Dufresne, The importance of the microbiome of the plant holobiont, New Phytol. 206 (2015) 1196– 1206. https://doi.org/10.1111/nph.13312.
[7] P. Kramer, P. Bressan, Humans as Superorganisms: How Microbes, Viruses, Imprinted Genes, and Other Selfish Entities Shape Our Behavior, Perspect Psychol Sci. 10 (2015) 464–481. https://doi.org/10.1177/1745691615583131.
[8] M. van de Guchte, H.M. Blottière, J. Doré, Humans as holobionts: implications for prevention and therapy, Microbiome. 6 (2018) 81. https://doi.org/10.1186/s40168-018- 0466-8.
[9] S. Khanna, P.K. Tosh, A Clinician’s Primer on the Role of the Microbiome in Human Health and Disease, Mayo Clinic Proceedings. 89 (2014) 107–114. https://doi.org/10.1016/j.mayocp.2013.10.011.
[10] A.K. Benson, S.A. Kelly, R. Legge, F. Ma, S.J. Low, J. Kim, M. Zhang, P.L. Oh, D. Nehrenberg, K. Hua, S.D. Kachman, E.N. Moriyama, J. Walter, D.A. Peterson, D. Pomp, Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors, Proc. Natl. Acad. Sci. U.S.A. 107 (2010) 18933–18938. https://doi.org/10.1073/pnas.1007028107.
[11] G.A. Ogunrinola, J.O. Oyewale, O.O. Oshamika, G.I. Olasehinde, The Human Microbiome and Its Impacts on Health, International Journal of Microbiology. 2020 (2020) 1–7. https://doi.org/10.1155/2020/8045646.
[12] R. Sender, S. Fuchs, R. Milo, Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans, Cell. 164 (2016) 337–340. https://doi.org/10.1016/j.cell.2016.01.013.
[13] W.R. Wikoff, A.T. Anfora, J. Liu, P.G. Schultz, S.A. Lesley, E.C. Peters, G. Siuzdak, Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites, Proc. Natl. Acad. Sci. U.S.A. 106 (2009) 3698–3703. https://doi.org/10.1073/pnas.0812874106.
[14] G.W. Tannock, What immunologists should know about bacterial communities of the human bowel, Semin Immunol. 19 (2007) 94–105. https://doi.org/10.1016/j.smim.2006.09.001.
[15] L. Dethlefsen, M. McFall-Ngai, D.A. Relman, An ecological and evolutionary perspective on human-microbe mutualism and disease, Nature. 449 (2007) 811–818. https://doi.org/10.1038/nature06245.
[16] R.E. Ley, D.A. Peterson, J.I. Gordon, Ecological and evolutionary forces shaping microbial diversity in the human intestine, Cell. 124 (2006) 837–848. https://doi.org/10.1016/j.cell.2006.02.017.
[17] F. Bäckhed, R.E. Ley, J.L. Sonnenburg, D.A. Peterson, J.I. Gordon, Host-bacterial mutualism in the human intestine, Science. 307 (2005) 1915–1920. https://doi.org/10.1126/science.1104816.
[18] B. Linz, F. Balloux, Y. Moodley, A. Manica, H. Liu, P. Roumagnac, D. Falush, C. Stamer, F. Prugnolle, S.W. van der Merwe, Y. Yamaoka, D.Y. Graham, E. Perez Trallero, T. Wadstrom, S. Suerbaum, M. Achtman, An African origin for the intimate association between humans and Helicobacter pylori, Nature. 445 (2007) 915–918. https://doi.org/10.1038/nature05562.
[19] J.L. Sonnenburg, F. Bäckhed, Diet–microbiota interactions as moderators of human metabolism, Nature. 535 (2016) 56–64. https://doi.org/10.1038/nature18846.
[20] D.A. Antonopoulos, S.M. Huse, H.G. Morrison, T.M. Schmidt, M.L. Sogin, V.B. Young, Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation, Infect Immun. 77 (2009) 2367–2375. https://doi.org/10.1128/IAI.01520-08.
[21] T.S. Postler, S. Ghosh, Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System, Cell Metabolism. 26 (2017) 110–130. https://doi.org/10.1016/j.cmet.2017.05.008.
[22] G. Sharon, N. Garg, J. Debelius, R. Knight, P.C. Dorrestein, S.K. Mazmanian, Specialized Metabolites from the Microbiome in Health and Disease, Cell Metabolism. 20 (2014) 719–730. https://doi.org/10.1016/j.cmet.2014.10.016.
[23] A. Hirschler, Ueber den Einfluss der Kohlehydrate und einiger anderer Körper der Fettsäurereihe auf die Eiweissfäulniss., Bchm. 10 (1886) 306–317. https://doi.org/10.1515/bchm1.1886.10.4.306.
[24] P.R. Cannon, The effects of diet on the intestinal flora, Journal of Infectious Diseases. 29 (1921) 369–385. https://doi.org/10.1093/infdis/29.5.369.
[25] H. Shapiro, J. Suez, E. Elinav, Personalized microbiome-based approaches to metabolic syndrome management and prevention, J Diabetes. 9 (2017) 226–236. https://doi.org/10.1111/1753-0407.12501.
[26] E. Salvucci, Microbiome, holobiont and the net of life, Crit Rev Microbiol. 42 (2016) 485–494. https://doi.org/10.3109/1040841X.2014.962478.





Comments (0)
There are no comments for this article. Be the first one to leave a message!