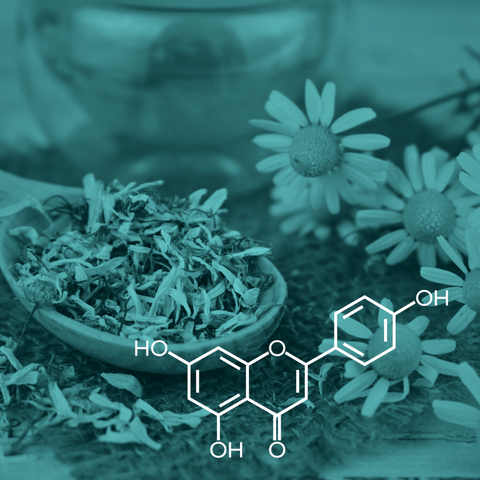
Apigenin, also known as 4′,5,7-trihydroxyflavone [1], is a natural chemical from the flavone group found in vegetables and fruits, including olives, onions, celery, oranges, and chamomile [2]. Plants belonging to the Asteraceae botanical family are the principal sources of this compound. It is synthesized in the phenylpropanoid pathway and is obtained from the phenylalanine and tyrosine amino acids [3]. Like other flavonoids, apigenin is known for its antioxidant properties [4]. It presents hypoglycemic [5], anti-inflammatory [6], and cytostatic and cytotoxic properties for various cancer cells [7]. Apigenin also has anti-apoptotic effects in myocardial ischemia [8].
Apigenin's low solubility in water and low bioavailability [9,10] limit its potential biological effects. Different delivery systems, including lysosomes, polymeric micelles, and nanosuspension, can improve its solubility [11-13], and a study in vitro suggests that injectable nanocapsules may be a suitable approach to achieve prolonged apigenin pharmacological activity [14].
In this article, we delve into the therapeutic potential of apigenin, including its mechanism of action, the benefits of apigenin, and current limitations in studies.
Apigenin mechanisms of action
Apigenin exhibits multiple functions in vitro through various mechanisms, highlighting its potential therapeutic applications. For instance, it modulates the expression of different genes, including CDKs, inducing cell cycle arrest at different stages (G1/S or G2/M phases) [15,16]. It regulates intrinsic apoptotic pathways by altering the mitochondrial membrane potential, which leads to the cytoplasmic release of cytochrome C and the activation of caspase-3, ultimately resulting in cell apoptosis [17]. Apigenin also modulates extrinsic apoptosis pathways via caspase-8 activation. In cancer cells, apigenin enhances apoptosis via increasing Bcl-2, Bax, STAT-3, and Akt proteins [18,19]. It demonstrates anti-inflammatory properties by reducing COX-2 activity, promoting the p38/MAPK and PI3K/Akt pathways, and preventing IKB degradation and NF-κB nuclear translocation [20,21]. Additionally, it inhibits the expression of pro-inflammatory cytokines, such as IL-6 [22], by blocking the phosphorylation of the p65 subunit and the subsequent activation of NF-κB [23].
Consequently, apigenin suppresses lipopolysaccharide-induced lethality [23]. Apigenin also presents antioxidant properties. It significantly increases the expression of GSH-synthase, catalase, and SOD, three antioxidant enzymes that counteract oxidative stress, and decreases the levels of lipid peroxidase [24]. It enhances the expression of phase II detoxifying enzyme-encoding genes by inhibiting the NADPH oxidase complex and its downstream target inflammatory genes while promoting the nuclear translocation of Nrf2 [25]. Finally, apigenin modulates signaling molecules in the three principal MAPK pathways: ERK, c-JNK, and p38 in human cells in vitro [26]. These findings underscore the multiplicity of apigenin's mechanisms of action, ranging from gene expression modulation and apoptosis regulation to anti-inflammatory and antioxidant effects, highlighting its diverse therapeutic potential across various cellular pathways.
Apigenin’s anti-diabetic properties
In vitro, apigenin inhibits the human α-amylase. This enzyme controls glucose assimilation, via a competitive mechanism by occupying its binding site [27-30], and the intestinal α-glucosidase, an enzyme involved in the absorption of glucose in the gastrointestinal tract [31-33]. It is a potent and selective inhibitor of the GLUT2 transporter, a protein that accounts for about 60% and 75% of total fructose and glucose uptake from the intestinal lumen, respectively, following a meal [34,35]. It also increases insulin secretion [36]. In high-fat diet-induced obese mice, apigenin treatment reduced glycemia and insulin resistance index and improved glucose tolerance [37]. Therefore, apigenin potentially appears as a good alternative to regulate postprandial carbohydrate absorption, alone or combined with anti-diabetic drugs for an enhanced formulation.
Apigenin’s antitumor properties
Apigenin shows cytostatic and cytotoxic effects on various cancer cells in vitro, including pancreatic cancer [38,39], breast cancer [40,41], ovarian cancer [42], lung cancer [43], and colorectal cancer [44] cell lines. In several experiments (in vitro and in vivo), apigenin showed antitumor properties with a significantly prolonged survival time and suppression of tumor growth [45,46]. This antitumor effect may result from apigenin’s ability to inhibit the activation of IKKα, which in turn does not activate NF-κB, as previously mentioned, and subsequent downregulation of genes involved in proliferation (such as the genes coding for cyclin D1 and COX-2), anti-apoptosis (Bcl-2 and Bcl-xL), and angiogenesis (VEGF)[17,38,39,47,48,48,49]. Therefore, apigenin shows potential as an antitumor substance.
The effects of apigenin on the liver
Apigenin may exert a protective effect on the liver via its antioxidant activity, including the enhanced expression of antioxidant enzymes (GSH-synthase, catalase, and SOD) and decreased levels of lipid peroxidase. After apigenin treatment of hepatoma cells, the expression of Nrf2-mediated antioxidant genes that protect against oxidative damage increases [50], suggesting that apigenin enhances the scavenging of reactive oxygen species. In addition, apigenin reduces hepatic inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. However, apigenin treatment at high doses triggers hepatic damage in mice with oxidative stress and apoptosis [51]. Therefore, further research is required to understand apigenin’s therapeutic potential for liver health.
The effects of apigenin on the cardiovascular system
Apigenin shows protective effects on the cardiovascular system. For instance, it enhances nitric oxide levels in the aorta, thus protecting the vascular endothelium [52]. By inhibiting the angiotensin-converting enzyme, apigenin reduces blood pressure [53]. It decreases atherogenesis via the induction of macrophage apoptosis and the subsequent reduction of inflammatory cytokines (TNF-α, IL-21, and IL-6) [54]. Several studies suggest that it may also protect the heart against ischemia and reperfusion injuries by up-regulating the expression of Bcl-2 and reducing the p38 mitogen-activated protein kinase signaling pathway [55-57]. These findings highlight apigenin's potential as a therapeutic agent to prevent and treat cardiovascular diseases.
Current limitations
Apigenin research has principally focused on in vitro experiments. Studies on animal models, including rats and mice, are limited, and available human data from clinical trials are reduced. Although great efforts have been made to tackle its low bioavailability, its high metabolic transformation, similar to other phytochemicals, remains an unsolved issue to demonstrate this substance’s structure-function relationship [1].
Conclusion
Apigenin exhibits numerous beneficial effects, including hypoglycemic, antioxidant, anti-inflammatory, antitumor, and hypotensive properties. However, most of these observed effects were observed in vitro. Further research, particularly in animal models and human studies, is needed to understand and confirm the therapeutic potential of apigenin.
Research has studied the effect of apigenin on cancer cells, particularly breast cancer cells, and has shown that apigenin induces apoptosis by apigenin in humans and inhibits cancer progression. The flavonoid apigenin has also demonstrated antitumor effects, with studies evaluating the impact of apigenin in patients with resected colorectal cancer to prevent recurrence. Additionally, combining apigenin with other flavonoids like quercetin and apigenin or apigenin and naringenin has been explored for enhanced therapeutic outcomes.
Despite promising findings, further research is needed to fully understand the chemopreventive potential of apigenin and its applications in clinical settings. Future studies should also investigate its dose-dependent hepatotoxicity and thoroughly evaluate its safety profile to ensure clinical applicability.
If you're a practitioner and interested in using apigenin as a sleep aid for your patients, check out Tro+ Somna, Troscriptions' most potent sleep formula yet. It's designed to tackle even the most challenging sleep disturbances. If your patients really struggle to fall asleep or stay asleep, before reaching for the prescription (or calling in a refill), Tro+ Somna may be their answer!
References
[1] Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A. et al. (2019) The Therapeutic Potential of Apigenin. International Journal of Molecular Sciences, 20, 1305. https://doi.org/10.3390/ijms20061305
[2] Wang, C., Feng, X., Li, W., Chen, L., Wang, X., Lan, Y. et al. (2024) Apigenin as an emerging hepatoprotective agent: current status and future perspectives. Frontiers in Pharmacology, 15, 1508060. https://doi.org/10.3389/fphar.2024.1508060
[3] Liu, S., Zheng, X., Luo, Z., Tang, C., Hu, Y., Peng, Q. et al. (2024) The synthesis and bioactivity of apigenin derivatives. Fitoterapia, 179, 106228. https://doi.org/10.1016/j.fitote.2024.106228
[4] Fidelis, Q.C., Faraone, I., Russo, D., Aragão Catunda-Jr, F.E., Vignola, L., De Carvalho, M.G. et al. (2019) Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: a source of bioactive compounds with multifunctional properties. Natural Product Research, 33, 1500–3. https://doi.org/10.1080/14786419.2017.1419227
[5] Villa-Rodriguez, J.A., Kerimi, A., Abranko, L., Tumova, S., Ford, L., Blackburn, R.S. et al. (2018) Acute metabolic actions of the major polyphenols in chamomile: an in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Scientific Reports, 8, 5471. https://doi.org/10.1038/s41598-018-23736-1
[6] Lim, R., Barker, G., Wall, C.A. and Lappas, M. (2013) Dietary phytophenols curcumin, naringenin and apigenin reduce infection-induced inflammatory and contractile pathways in human placenta, foetal membranes and myometrium. MHR: Basic Science of Reproductive Medicine, 19, 451–62. https://doi.org/10.1093/molehr/gat015
[7] Zhou, X., Wang, F., Zhou, R., Song, X. and Xie, M. (2017) Apigenin: A current review on its beneficial biological activities. Journal of Food Biochemistry, 41, e12376. https://doi.org/10.1111/jfbc.12376
[8] Zhou, Z., Zhang, Y., Lin, L. and Zhou, J. (2018) Apigenin suppresses the apoptosis of H9C2 rat cardiomyocytes subjected to myocardial ischemia‑reperfusion injury via upregulation of the PI3K/Akt pathway. Molecular Medicine Reports,. https://doi.org/10.3892/mmr.2018.9115
[9] Meyer, H., Bolarinwa, A., Wolfram, G. and Linseisen, J. (2006) Bioavailability of Apigenin from Apiin-Rich Parsley in Humans. Annals of Nutrition and Metabolism, 50, 167–72. https://doi.org/10.1159/000090736
[10] Borges, G., Fong, R.Y., Ensunsa, J.L., Kimball, J., Medici, V., Ottaviani, J.I. et al. (2022) Absorption, distribution, metabolism and excretion of apigenin and its glycosides in healthy male adults. Free Radical Biology and Medicine, 185, 90–6. https://doi.org/10.1016/j.freeradbiomed.2022.04.007
[11] Zhai, Y., Guo, S., Liu, C., Yang, C., Dou, J., Li, L. et al. (2013) Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 429, 24–30. https://doi.org/10.1016/j.colsurfa.2013.03.051
[12] Ding, B., Chen, H., Wang, C., Zhai, Y. and Zhai, G. (2013) Preparation and <I>In Vitro</I> Evaluation of Apigenin Loaded Lipid Nanocapsules. Journal of Nanoscience and Nanotechnology, 13, 6546–52. https://doi.org/10.1166/jnn.2013.7763
[13] Al Shaal, L., Shegokar, R. and Müller, R.H. (2011) Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. International Journal of Pharmaceutics, 420, 133–40. https://doi.org/10.1016/j.ijpharm.2011.08.018
[14] Karim, R., Palazzo, C., Laloy, J., Delvigne, A.-S., Vanslambrouck, S., Jerome, C. et al. (2017) Development and evaluation of injectable nanosized drug delivery systems for apigenin. International Journal of Pharmaceutics, 532, 757–68. https://doi.org/10.1016/j.ijpharm.2017.04.064
[15] Takagaki, N., Sowa, Y., Oki, T., Nakanishi, R., Yogosawa, S. and Sakai, T. (2005) Apigenin induces cell cycle arrest and p21/WAF1 expression in a p53-independent pathway. International Journal of Oncology,. https://doi.org/10.3892/ijo.26.1.185
[16] Maggioni, D., Garavello, W., Rigolio, R., Pignataro, L., Gaini, R. and Nicolini, G. (2013) Apigenin impairs oral squamous cell carcinoma growth in vitro inducing cell cycle arrest and apoptosis. International Journal of Oncology, 43, 1675–82. https://doi.org/10.3892/ijo.2013.2072
[17] Seo, H.-S., Ku, J.M., Choi, H.-S., Woo, J.-K., Jang, B.-H., Shin, Y.C. et al. (2014) Induction of caspase-dependent apoptosis by apigenin by inhibiting STAT3 signaling in HER2-overexpressing MDA-MB-453 breast cancer cells. Anticancer Research, 34, 2869–82.
[18] Seo, H.-S., Choi, H.-S., Kim, S.-R., Choi, Y.K., Woo, S.-M., Shin, I. et al. (2012) Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Molecular and Cellular Biochemistry, 366, 319–34. https://doi.org/10.1007/s11010-012-1310-2
[19] Karmakar, S., Davis, K.A., Choudhury, S.R., Deeconda, A., Banik, N.L. and Ray, S.K. (2009) Bcl-2 inhibitor and apigenin worked synergistically in human malignant neuroblastoma cell lines and increased apoptosis with activation of extrinsic and intrinsic pathways. Biochemical and Biophysical Research Communications, 388, 705–10. https://doi.org/10.1016/j.bbrc.2009.08.071
[20] Lee, J.-H., Zhou, H.Y., Cho, S.Y., Kim, Y.S., Lee, Y.S. and Jeong, C.S. (2007) Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Archives of Pharmacal Research, 30, 1318–27. https://doi.org/10.1007/BF02980273
[21] Huang, C.-H., Kuo, P.-L., Hsu, Y.-L., Chang, T.-T., Tseng, H.-I., Chu, Y.-T. et al. (2010) The Natural Flavonoid Apigenin Suppresses Th1- and Th2-Related Chemokine Production by Human Monocyte THP-1 Cells Through Mitogen-Activated Protein Kinase Pathways. Journal of Medicinal Food, 13, 391–8. https://doi.org/10.1089/jmf.2009.1229
[22] Rezai-Zadeh, K., Ehrhart, J., Bai, Y., Sanberg, P.R., Bickford, P., Tan, J. et al. (2008) Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. Journal of Neuroinflammation, 5, 41. https://doi.org/10.1186/1742-2094-5-41
[23] Nicholas, C., Batra, S., Vargo, M.A., Voss, O.H., Gavrilin, M.A., Wewers, M.D. et al. (2007) Apigenin Blocks Lipopolysaccharide-Induced Lethality In Vivo and Proinflammatory Cytokines Expression by Inactivating NF-κB through the Suppression of p65 Phosphorylation. The Journal of Immunology, 179, 7121–7. https://doi.org/10.4049/jimmunol.179.10.7121
[24] Telange, D.R., Patil, A.T., Pethe, A.M., Fegade, H., Anand, S. and Dave, V.S. (2017) Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. European Journal of Pharmaceutical Sciences, 108, 36–49. https://doi.org/10.1016/j.ejps.2016.12.009
[25] Kashyap, D., Sharma, A., Tuli, H.S., Sak, K., Garg, V.K., Buttar, H.S. et al. (2018) Apigenin: A natural bioactive flavone-type molecule with promising therapeutic function. Journal of Functional Foods, 48, 457–71. https://doi.org/10.1016/j.jff.2018.07.037
[26] Peng, Q., Deng, Z., Pan, H., Gu, L., Liu, O. and Tang, Z. (2017) Mitogen-activated protein kinase signaling pathway in oral cancer (Review). Oncology Letters,. https://doi.org/10.3892/ol.2017.7491
[27] Brayer, G.D., Sidhu, G., Maurus, R., Rydberg, E.H., Braun, C., Wang, Y. et al. (2000) Subsite Mapping of the Human Pancreatic α-Amylase Active Site through Structural, Kinetic, and Mutagenesis Techniques,. Biochemistry, 39, 4778–91. https://doi.org/10.1021/bi9921182
[28] Lo Piparo, E., Scheib, H., Frei, N., Williamson, G., Grigorov, M. and Chou, C.J. (2008) Flavonoids for Controlling Starch Digestion: Structural Requirements for Inhibiting Human α-Amylase. Journal of Medicinal Chemistry, 51, 3555–61. https://doi.org/10.1021/jm800115x
[29] Williams, L.K., Zhang, X., Caner, S., Tysoe, C., Nguyen, N.T., Wicki, J. et al. (2015) The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nature Chemical Biology, 11, 691–6. https://doi.org/10.1038/nchembio.1865
[30] Williams, L.K., Li, C., Withers, S.G. and Brayer, G.D. (2012) Order and Disorder: Differential Structural Impacts of Myricetin and Ethyl Caffeate on Human Amylase, an Antidiabetic Target. Journal of Medicinal Chemistry, 55, 10177–86. https://doi.org/10.1021/jm301273u
[31] Flores-Bocanegra, L., González-Andrade, M., Bye, R., Linares, E. and Mata, R. (2017) α-Glucosidase Inhibitors from Salvia circinata. Journal of Natural Products, 80, 1584–93. https://doi.org/10.1021/acs.jnatprod.7b00155
[32] Li, H., Song, F., Xing, J., Tsao, R., Liu, Z. and Liu, S. (2009) Screening and structural characterization of α -glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MS n and SORI-CID FTICR MS. Journal of the American Society for Mass Spectrometry, 20, 1496–503. https://doi.org/10.1016/j.jasms.2009.04.003
[33] Zhang, X., Liu, Z., Bi, X., Liu, J., Li, W. and Zhao, Y. (2013) Flavonoids and its derivatives from Callistephus chinensis flowers and their inhibitory activities against alpha-glucosidase. EXCLI Journal, 12, 956–66.
[34] Gouyon, F., Caillaud, L., Carrière, V., Klein, C., Dalet, V., Citadelle, D. et al. (2003) Simple‐sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2‐null mice. The Journal of Physiology, 552, 823–32. https://doi.org/10.1113/jphysiol.2003.049247
[36] Pamunuwa, G., Karunaratne, D.N. and Waisundara, V.Y. (2016) Antidiabetic Properties, Bioactive Constituents, and Other Therapeutic Effects of Scoparia dulcis. Razzaque MS, editor. Evidence-Based Complementary and Alternative Medicine, 2016, 8243215. https://doi.org/10.1155/2016/8243215
[37] Jung, U., Cho, Y.-Y. and Choi, M.-S. (2016) Apigenin Ameliorates Dyslipidemia, Hepatic Steatosis and Insulin Resistance by Modulating Metabolic and Transcriptional Profiles in the Liver of High-Fat Diet-Induced Obese Mice. Nutrients, 8, 305. https://doi.org/10.3390/nu8050305
[38] Johnson, J.L. and De Mejia, E.G. (2013) Flavonoid apigenin modified gene expression associated with inflammation and cancer and induced apoptosis in human pancreatic cancer cells through inhibition of GSK ‐3β/ NF ‐κ B signaling cascade. Molecular Nutrition & Food Research, 57, 2112–27. https://doi.org/10.1002/mnfr.201300307
[39] Wu, D.-G., Yu, P., Li, J.-W., Jiang, P., Sun, J., Wang, H.-Z. et al. (2014) Apigenin potentiates the growth inhibitory effects by IKK-β-mediated NF-κB activation in pancreatic cancer cells. Toxicology Letters, 224, 157–64. https://doi.org/10.1016/j.toxlet.2013.10.007
[40] Bai, H., Jin, H., Yang, F., Zhu, H. and Cai, J. (2014) Apigenin induced MCF-7 cell apoptosis-associated reactive oxygen species: Apigenin Induced MCF-7 Cell Apoptosis. Scanning, 36, 622–31. https://doi.org/10.1002/sca.21170
[41] Harrison, M.E., Power Coombs, M.R., Delaney, L.M. and Hoskin, D.W. (2014) Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Experimental and Molecular Pathology, 97, 211–7. https://doi.org/10.1016/j.yexmp.2014.07.006
[42] Suh, Y.-A., Jo, S.-Y., Lee, H.-Y. and Lee, C. (2015) Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells. International Journal of Oncology, 46, 1405–11. https://doi.org/10.3892/ijo.2014.2808
[43] Lee, Y.-M., Lee, G., Oh, T.-I., Kim, B.M., Shim, D.-W., Lee, K.-H. et al. (2016) Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. International Journal of Oncology, 48, 399–408. https://doi.org/10.3892/ijo.2015.3243
[44] Xu, M., Wang, S., Song, Y., Yao, J., Huang, K. and Zhu, X. (2016) Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β-catenin signaling pathway. Oncology Letters, 11, 3075–80. https://doi.org/10.3892/ol.2016.4331
[45] Chen, D., Landis-Piwowar, K.R., Chen, M.S. and Dou, Q.P. (2007) Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Research, 9, R80. https://doi.org/10.1186/bcr1797
[46] Shukla, S., Shankar, E., Fu, P., MacLennan, G.T. and Gupta, S. (2015) Suppression of NF-κB and NF-κB-Regulated Gene Expression by Apigenin through IκBα and IKK Pathway in TRAMP Mice. Gautam S, editor. PLOS ONE, 10, e0138710. https://doi.org/10.1371/journal.pone.0138710
[47] Shukla, S., Fu, P. and Gupta, S. (2014) Apigenin induces apoptosis by targeting inhibitor of apoptosis proteins and Ku70–Bax interaction in prostate cancer. Apoptosis, 19, 883–94. https://doi.org/10.1007/s10495-014-0971-6
[48] Shukla, S., Kanwal, R., Shankar, E., Datt, M., Chance, M.R., Fu, P. et al. (2015) Apigenin blocks IKKα activation and suppresses prostate cancer progression. Oncotarget, 6, 31216–32. https://doi.org/10.18632/oncotarget.5157
[49] Wang, Y., Xu, Y.S., Yin, L.H., Xu, L.N., Peng, J.Y., Zhou, H. et al. (2013) Synergistic anti-glioma effect of Hydroxygenkwanin and Apigenin in vitro. Chemico-Biological Interactions, 206, 346–55. https://doi.org/10.1016/j.cbi.2013.10.009
[50] Paredes‐Gonzalez, X., Fuentes, F., Jeffery, S., Saw, C.L., Shu, L., Su, Z. et al. (2015) Induction of NRF2‐mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharmaceutics & Drug Disposition, 36, 440–51. https://doi.org/10.1002/bdd.1956
[51] Singh, P., Mishra, S.K., Noel, S., Sharma, S. and Rath, S.K. (2012) Acute Exposure of Apigenin Induces Hepatotoxicity in Swiss Mice. Ahmad A, editor. PLoS ONE, 7, e31964. https://doi.org/10.1371/journal.pone.0031964
[52] Jin, B., Qian, L., Chen, S., Li, J., Wang, H., Bruce, I.C. et al. (2009) Apigenin protects endothelium-dependent relaxation of rat aorta against oxidative stress. European Journal of Pharmacology, 616, 200–5. https://doi.org/10.1016/j.ejphar.2009.06.020
[53] Salah, A.M., Dongmo, A.B., Kamanyi, A., Bopelet, M. and Wagner, H. (2001) Angiotensin-Converting Enzyme-Inhibitory Effect by Ruellia praetermissa. Pharmaceutical Biology, 39, 16–9. https://doi.org/10.1076/phbi.39.1.16.5942
[54] Wang, Q., Zeng, P., Liu, Y., Wen, G., Fu, X. and Sun, X. (2015) Inhibition of autophagy ameliorates atherogenic inflammation by augmenting apigenin-induced macrophage apoptosis. International Immunopharmacology, 27, 24–31. https://doi.org/10.1016/j.intimp.2015.04.018
[55] Chen, C., He, H., Luo, Y., Zhou, M., Yin, D. and He, M. (2016) Involvement of Bcl-2 Signal Pathway in the Protective Effects of Apigenin on Anoxia/Reoxygenation-induced Myocardium Injury: Journal of Cardiovascular Pharmacology, 67, 152–63. https://doi.org/10.1097/FJC.0000000000000331
[56] Hu, J., Li, Z., Xu, L., Sun, A., Fu, X., Zhang, L. et al. (2015) Protective Effect of Apigenin on Ischemia/Reperfusion Injury of the Isolated Rat Heart. Cardiovascular Toxicology, 15, 241–9. https://doi.org/10.1007/s12012-014-9290-y
[57] Yang, X., Yang, J., Hu, J., Li, X., Zhang, X. and Li, Z. (2015) Apigenin attenuates myocardial ischemia/reperfusion injury via the inactivation of p38 mitogen-activated protein kinase. Molecular Medicine Reports, 12, 6873–8. https://doi.org/10.3892/mmr.2015.4293




Comments (0)
There are no comments for this article. Be the first one to leave a message!