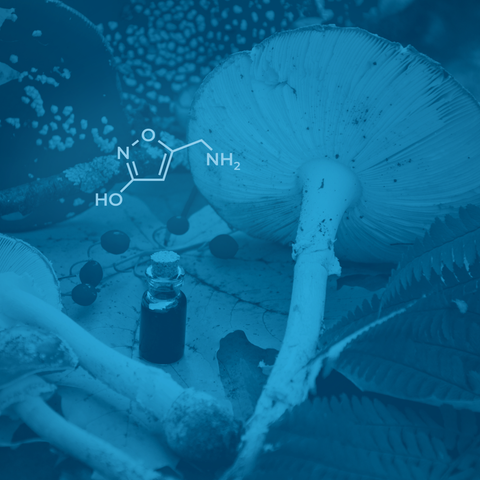
Agarin, also known as muscimol, pantherine, or (5-aminomethyl)-isoxazol-3-ol, is the main psychoactive compound produced by the fly agaric or Amanita muscaria mushroom. Agarin is chemically very similar to gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain. GABA is synthesized from glutamate by the enzyme glutamate decarboxylase with pyridoxal phosphate (vitamin B6) as a cofactor. Increasing GABA production or intake improves sleep quality, decreases anxiety, protects the brain (neuroprotective), suppresses neurodegeneration, and decreases blood pressure. You can read more about it here.
Agarin acts as a GABA-mimetic compound, directly binding to GABAA receptors, and mimics the inhibitory effects of GABA. In the central nervous system, GABA binding to GABAA receptors induces both phasic inhibition [1,2], by transiently reducing postsynaptic neuron excitability, and tonic inhibition, which provides continuous regulation of neural excitation through long-term hyperpolarization, playing a key role in synaptic plasticity and neurogenesis [3,4].
Unlike other GABAergic drugs that allosterically modulate these receptors, agarin binds directly to the GABA binding site at the β+/α− interface to exert potent and selective agonist effects [5-8]. After enteral or parenteral administration, agarin rapidly crosses the blood-brain barrier to reach the central nervous system and displays depressant, sedative-hypnotic, and hallucinogenic effects [9].
In this article, we'll dive more into agarin's interactions with GABA and its uses in research. You can read more about agarin here and here. You can also read more about the fly agaric mushroom's other bioactive compounds here.
Agarin use in research
Agarin for studying GABAA receptors
Radiolabeled agarin has been widely used in research to investigate the expression and distribution of GABA receptors in the brain and other tissues [5,10]. For example, agarin has revealed an increased expression of GABAA receptors in the prefrontal cortex of patients with schizophrenia [11], and a loss in brain regions involved in face processing in patients with autism [12].
Studies on the modulation of agarin binding in the presence of GABAergic drugs have provided insights about the respective binding sites of these drugs on GABAA receptors [13-16].
Agarin structure used to derive GABAergic modulators
Agarin chemical backbone has served as a template for designing GABA signaling modulators [17]. These derived analogues include 4,5,6,7-tetrahydroisoxazolo(5,4-c)pyridin-3-ol (also known as THIP, or gaboxadol); 4,5,6,7-tetrahydroisoxazolo(4,5-c)pyridin-3-ol (THPO); iso-THIP; and 5-(4-piperidyl)-3-hydroxyisoxazole (4-PIOL) [18]. Although structurally similar, these compounds interact with the GABAergic system very differently and display distinct pharmacological profiles [19]. For example, THPO inhibits GABA uptake [20] through the glial transport system [21], while THIP is a potent GABAA receptor agonist [22-24].
Agarin and its potential therapeutic uses
Agarin holds promise for the therapy of neurodegenerative disorders such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). It may also show beneficial effects in treating epilepsy, seizures, and nerve injury-related neuropathic pain.
Essential tremors and Parkinson’s disease
PD is the second most common neurodegenerative disorder after AD. It mainly affects the elderly population and causes bradykinesia, akinesia, tremors, and rigidity. The etiology of the disease remains unknown, although several factors, including α-synuclein aggregates, neurotransmitter imbalances, oxidative stress, local inflammation, and dopaminergic neurodegeneration in the substantia nigra, have been identified [25,26].
Agarin, as a GABAA receptor agonist, exerts great inhibitory properties and was tested in patients with essential tremors. One study reported suppression of the tremors after agarin microinjections without influencing the speech and movements of the patients [27]. Another study demonstrated that agarin microinjections into a region with tremor-related activity resulted in the suppression of limb tremors in two patients with PD [28]. Thus, targeted inhibition of specific brain regions with agarin or other GABA analogs could potentially be used to treat essential tremors and PD-related tremors.
Alzheimer’s disease
AD is the most common cause of dementia worldwide, accounting for about 60-80% of cases with an estimated 5.8 million Americans age 65 and older living with the disease in 2020. The disease results from an accumulation of amyloid plaques in the brain and an aggregation of abnormal tau proteins in neurofibrillary tangles inside patients’ neurons [29,30]. It causes memory loss, confusion, changes in personality, and a gradual loss of independence [31]. The GABAergic system regulates the learning and memory processes and evidence suggests that it is involved in the pathophysiology of AD via a decrease in functional GABAA receptors [32], deficits in inhibitory interneurons [33], glial cell overactivation, and neurotransmission impairment, leading to neuroinflammation and neurodegeneration [34].
A study in cultured cortical neurons from rats showed that chronic modulation of GABAA receptors with agarin was able to reduce β-amyloid neurotoxicity. This effect was inhibited by the bicuculline, a GABAA receptor antagonist, suggesting that GABAA receptor activation is necessary for agarin's protective effects [35]. Other studies have demonstrated that treatment with agarin exhibited anti-inflammatory effects, normalized GABA expression, enhanced memory, and improved learning abilities in a rat model of streptozocin-induced AD [34,36].
Taken together, these findings suggest that enhancing GABAergic processes with agarin may help prevent early memory decline in AD.
Epilepsy and seizures
Agarin has demonstrated significant potential for treating epilepsy and seizures in rats and monkeys. Acute injection of agarin into the subthalamic nucleus, a brain region involved in controlling various seizure types, produced potent anti-seizure effects in rat epilepsy and seizure models [37-39]. Building on these findings, a recent study in rats explored chronic convection-enhanced delivery of agarin into the subthalamic nucleus over three weeks, revealing sustained anti-seizure effects with a dose-dependent increase in responders [40]. These results suggest that agarin could be a promising long-term treatment for managing epilepsy and seizures through targeted brain delivery.
Nerve injury-related neuropathic pain
Agarin’s GABAergic properties make it a potential candidate for pain treatment. A recent meta-analysis investigated agarin efficacy in reducing nerve injury-related neuropathic pain and included 22 studies from rats and mice. Pooled data analysis showed that agarin is effective in reducing mechanical allodynia, mechanical hyperalgesia, and thermal hyperalgesia. In addition, agarin’s analgesic effects were observed 15 minutes after administration and lasted for up to 3 hours [41]. These findings suggest that agarin may be a promising candidate for the treatment of nerve injury-related neuropathic pain.
Safety profile
As with alcohol or benzodiazepines, agarin is a GABAA receptor agonist. As such, it shows inhibitory effects on the central nervous system. Agarin can cause nausea, dizziness, tiredness, and hallucinations at high doses [42]. To assess the toxicity of agarin from direct mushroom consumption, a retrospective study reviewed all cases of agarin-containing mushroom ingestions reported to a poison control center over 14 years. All patients reported gastrointestinal symptoms with varying degrees of sedation and agitation [43]. Agarin does not have the same toxic properties as ibotenic acid, another bioactive compound present in the fly agaric mushroom. However, there is currently no available toxicology data for agarin alone in humans.
Reports of mushroom intoxication linked to the fly agaric mushroom have been reported in humans; however, this is infrequent due to the distinct appearance of Amanita muscaria, which sets it apart from other edible mushrooms.
Given the above, it is imperative to consult with your healthcare provider and use trusted sources before deciding to take agarin or agarin supplements.
Conclusion
Agarin's unique properties as a GABAA receptor agonist make it a valuable tool in research and therapeutic development. Several studies have highlighted its potential for managing conditions such as Parkinson's disease, Alzheimer's disease, epilepsy, seizures, and nerve injury-related neuropathic pain. However, while agarin's efficacy is supported by preclinical data, further research is needed to fully understand its safety profile and therapeutic potential in clinical settings.
If you're interested in giving agarin a try, then check out Tro Zzz, our buccal troche formulated for sleep. It harnesses the combined power of science, nature, and your GABA system to help you achieve the sleep you've been longing for!
References
[1] Farrant, M. and Nusser, Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Reviews Neuroscience, 6, 215–29. https://doi.org/10.1038/nrn1625
[2] Li, Y., Sun, H., Chen, Z., Xu, H., Bu, G. and Zheng, H. (2016) Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Frontiers in Aging Neuroscience, 8. https://doi.org/10.3389/fnagi.2016.00031
[3] Ge, S., Goh, E.L.K., Sailor, K.A., Kitabatake, Y., Ming, G. and Song, H. (2006) GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature, 439, 589–93. https://doi.org/10.1038/nature04404
[4] Duveau, V., Laustela, S., Barth, L., Gianolini, F., Vogt, K.E., Keist, R. et al. (2011) Spatiotemporal specificity of GABAA receptor-mediated regulation of adult hippocampal neurogenesis: Adult neurogenesis regulation by GABAA receptors. European Journal of Neuroscience, 34, 362–73. https://doi.org/10.1111/j.1460-9568.2011.07782.x
[6] Müller, V., Ernst, M., Baykuchkarova, A., Koniuszewski, F., Bampali, K., Seidel, T. et al. (2024) Probes for the heterogeneity of muscimol binding sites in rat brain. Frontiers in Pharmacology, 15, 1368527. https://doi.org/10.3389/fphar.2024.1368527
[7] Deng, L., Ransom, R.W. and Olsen, R.W. (1986) [3H]Muscimol photolabels the γ-aminobutyric acid receptor binding site on a peptide subunit distinct from that labeled with benzodiazepines. Biochemical and Biophysical Research Communications, 138, 1308–14. https://doi.org/10.1016/S0006-291X(86)80425-9
[10] Chandra, D., Halonen, L.M., Linden, A.-M., Procaccini, C., Hellsten, K., Homanics, G.E. et al. (2010) Prototypic GABAA Receptor Agonist Muscimol Acts Preferentially Through Forebrain High-Affinity Binding Sites. Neuropsychopharmacology, 35, 999–1007. https://doi.org/10.1038/npp.2009.203
[11] Verdurand, M., Fillman, S.G., Shannon Weickert, C. and Zavitsanou, K. (2013) Increases in [3H]Muscimol and [3H]Flumazenil Binding in the Dorsolateral Prefrontal Cortex in Schizophrenia Are Linked to α4 and γ2S mRNA Levels Respectively. Rudolph U, editor. PLoS ONE, 8, e52724. https://doi.org/10.1371/journal.pone.0052724
[12] Oblak, A.L., Gibbs, T.T. and Blatt, G.J. (2011) Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Research, 1380, 218–28. https://doi.org/10.1016/j.brainres.2010.09.021
[15] Kirkness, E.F. and Turner, A.J. (1988) The stimulatory effects of secobarbital and pregnanolone on the GABAA receptor can be blocked selectively. European Journal of Pharmacology, 150, 385–8. https://doi.org/10.1016/0014-2999(88)90024-6
[16] Peters, J.A., Kirkness, E.F., Callachan, H., Lambert, J.J. and Turner, A.J. (1988) Modulation of the GABA A receptor by depressant barbiturates and pregnane steroids. British Journal of Pharmacology, 94, 1257–69. https://doi.org/10.1111/j.1476-5381.1988.tb11646.x
[18] Krall, J., Balle, T., Krogsgaard-Larsen, N., Sørensen, T.E., Krogsgaard-Larsen, P., Kristiansen, U. et al. (2015) GABAA Receptor Partial Agonists and Antagonists: Structure, Binding Mode, and Pharmacology. Advances in Pharmacology, Elsevier. p. 201–27. https://doi.org/10.1016/bs.apha.2014.10.003
[19] Okhovat, A., Cruces, W., Docampo-Palacios, M.L., Ray, K.P. and Ramirez, G.A. (2023) Psychoactive Isoxazoles, Muscimol, and Isoxazole Derivatives from the Amanita (Agaricomycetes) Species: Review of New Trends in Synthesis, Dosage, and Biological Properties. International Journal of Medicinal Mushrooms, 25, 1–10. https://doi.org/10.1615/IntJMedMushrooms.2023049458
[20] Krogsgaard‐Larsen, P., Johnston, G.A.R., Curtis, D.R., Game, C.J.A. and McCulloch, R.M. (1975) Structure and biological activity of a series of conformationally restricted analogues of GABA. Journal of Neurochemistry, 25, 803–9. https://doi.org/10.1111/j.1471-4159.1975.tb04411.x
[21] Schousboe, A., Larsson, O.M., Hertz, L. and Krogsgaard-Larsen, P. (1981) Heterocyclic GABA analogues as selective inhibitors of astroglial GABA uptake. Advances in Biochemical Psychopharmacology, 29, 135–41.
[22] Krogsgaard-Larsen, P., Johnston, G.A.R., Lodge, D. and Curtis, D.R. (1977) A new class of GABA agonist. Nature, 268, 53–5. https://doi.org/10.1038/268053a0
[23] Krogsgaard‐Larsen, P. and Johnston, G.A.R. (1978) Structure-activity studies on the inhibition of GABA binding to rat brain membranes by muscimol and related compounds. Journal of Neurochemistry, 30, 1377–82. https://doi.org/10.1111/j.1471-4159.1978.tb10469.x
[24] Krogsgaard‐Larsen, P., Hjeds, H., Curtis, D.R., Lodge, D. and Johnston, G.A.R. (1979) Dihydromuscimol, thiomuscimol and related heterocyclic compounds as GABA analogues. Journal of Neurochemistry, 32, 1717–24. https://doi.org/10.1111/j.1471-4159.1979.tb02284.x
[25] Kondeva-Burdina, M., Voynova, M., Shkondrov, A., Aluani, D., Tzankova, V. and Krasteva, I. (2019) Effects of Amanita muscaria extract on different in vitro neurotoxicity models at sub-cellular and cellular levels. Food and Chemical Toxicology, 132, 110687. https://doi.org/10.1016/j.fct.2019.110687
[26] Singh, A., Sinha, S. and Singh, N.K. (2024) Dietary Natural Flavonoids: Intervention for MAO ‐B Against Parkinson’s Disease. Chemical Biology & Drug Design, 104, e14619. https://doi.org/10.1111/cbdd.14619
[27] Pahapill, P.A., Levy, R., Dostrovsky, J.O., Davis, K.D., Rezai, A.R., Tasker, R.R. et al. (1999) Tremor arrest with thalamic microinjections of muscimol in patients with essential tremor. Annals of Neurology, 46, 249–52. https://doi.org/10.1002/1531-8249(199908)46:2<249::aid-ana15>3.0.co;2-c
[31] Li, Y.-B., Fu, Q., Guo, M., Du, Y., Chen, Y. and Cheng, Y. (2024) MicroRNAs: pioneering regulators in Alzheimer’s disease pathogenesis, diagnosis, and therapy. Translational Psychiatry, 14, 367. https://doi.org/10.1038/s41398-024-03075-8
[32] Limon, A., Reyes-Ruiz, J.M. and Miledi, R. (2012) Loss of functional GABA A receptors in the Alzheimer diseased brain. Proceedings of the National Academy of Sciences, 109, 10071–6. https://doi.org/10.1073/pnas.1204606109
[33] Verret, L., Mann, E.O., Hang, G.B., Barth, A.M.I., Cobos, I., Ho, K. et al. (2012) Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell, 149, 708–21. https://doi.org/10.1016/j.cell.2012.02.046
[34] Pilipenko, V., Narbute, K., Beitnere, U., Rumaks, J., Pupure, J., Jansone, B. et al. (2018) Very low doses of muscimol and baclofen ameliorate cognitive deficits and regulate protein expression in the brain of a rat model of streptozocin-induced Alzheimer’s disease. European Journal of Pharmacology, 818, 381–99. https://doi.org/10.1016/j.ejphar.2017.11.012
[35] Lee, B.Y., Ban, J.Y. and Seong, Y.H. (2005) Chronic stimulation of GABAA receptor with muscimol reduces amyloid β protein (25–35)-induced neurotoxicity in cultured rat cortical cells. Neuroscience Research, 52, 347–56. https://doi.org/10.1016/j.neures.2005.04.008
[36] Pilipenko, V., Pupure, J., Rumaks, J., Beitnere, U., Dzirkale, Z., Skumbins, R. et al. (2015) GABAA agonist muscimol ameliorates learning/memory deficits in streptozocin-induced Alzheimer’s disease non-transgenic rat model. SpringerPlus, 4, P36, 2193-1801-4-S1-P36. https://doi.org/10.1186/2193-1801-4-S1-P36
[37] Kohane, D.S., Holmes, G.L., Chau, Y., Zurakowski, D., Langer, R. and Cha, B.H. (2002) Effectiveness of Muscimol‐containing Microparticles against Pilocarpine‐induced Focal Seizures. Epilepsia, 43, 1462–8. https://doi.org/10.1046/j.1528-1157.2002.11202.x
[39] Ludvig, N., Baptiste, S.L., Tang, H.M., Medveczky, G., Von Gizycki, H., Charchaflieh, J. et al. (2009) Localized transmeningeal muscimol prevents neocortical seizures in rats and nonhuman primates: Therapeutic implications. Epilepsia, 50, 678–93. https://doi.org/10.1111/j.1528-1167.2008.01914.x
[40] Gernert, M., MacKeigan, D., Deking, L., Kaczmarek, E. and Feja, M. (2023) Acute and chronic convection-enhanced muscimol delivery into the rat subthalamic nucleus induces antiseizure effects associated with high responder rates. Epilepsy Research, 190, 107097. https://doi.org/10.1016/j.eplepsyres.2023.107097
[41] Ramawad, H.A., Paridari, P., Jabermoradi, S., Gharin, P., Toloui, A., Safari, S. et al. (2023) Muscimol as a treatment for nerve injury-related neuropathic pain: a systematic review and meta-analysis of preclinical studies. The Korean Journal of Pain, 36, 425–40. https://doi.org/10.3344/kjp.23161
[42] Leas, E.C., Satybaldiyeva, N., Kepner, W., Yang, K.H., Harati, R.M., Corroon, J. et al. (2024) Need for a Public Health Response to the Unregulated Sales of Amanita muscaria Mushrooms. American Journal of Preventive Medicine, 67, 458–63. https://doi.org/10.1016/j.amepre.2024.05.006
[43] Moss, M.J. and Hendrickson, R.G. (2019) Toxicity of muscimol and ibotenic acid containing mushrooms reported to a regional poison control center from 2002–2016. Clinical Toxicology, 57, 99–103. https://doi.org/10.1080/15563650.2018.1497169




Comments (0)
There are no comments for this article. Be the first one to leave a message!