This article was originally posted on Sept 7, 2023 and updated on Jun 20, 2024.
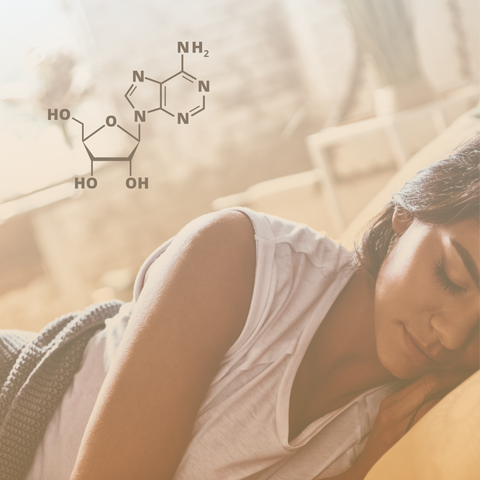
Adenosine, a naturally occurring nucleoside in the brain, contributes to energy metabolism, nucleic acid structure, cell signaling, and neurotransmission, among many other things. It binds to four subtypes of adenosine receptors (ARs), A1, A2A, A2B, and A3, all of which can be activated by extracellular adenosine [1]. The tissue-varying expression pattern of ARs and their different binding affinities with adenosine confer many functions to adenosine signaling in the human body [2,3].
ARs are widely expressed in the central nervous system (spinal cord and brain) and peripheral tissues [1,4]. In the brain, adenosine is a neurotransmitter that participates in neuromodulation, pain modulation, and even sleep regulation. This molecule also exhibits neuroprotective effects and, with its receptors, may be associated with several diseases.
A1 and A2A receptors are broadly distributed throughout the brain. A1Rs are uniformly expressed in brain tissue and exert an inhibitory effect at synapses by activating potassium and chloride channels and inhibiting P- and N-type voltage-gated calcium channels. In contrast, A2ARs are primarily expressed in the striatum, nucleus accumbens, and thalamus [5], where they activate adenylyl cyclase and promote cAMP production. A2A and A1Rs can form functional heteromers together [6] and with other excitatory receptors, such as those in the dopamine [7,8] and glutamate systems [9].
In this article, we'll take a deep dive into all things adenosine, including its interactions with caffeine and possible supplementation. If you'd like to read even more about adenosine's capabilities, take a look at our article on adenosine for skin!
Adenosine and sleep regulation
The concentration of adenosine varies across brain regions, leading to varied mechanisms in the regulation of sleep, primarily via A1 and A2 receptors [10]. For example, in the posterior hypothalamus, endogenous adenosine inhibits histaminergic signaling via A1R, promoting non-rapid eye movement sleep [11]. Conversely, in the ventrolateral preoptic area, adenosine inhibits GABAergic signaling to facilitate sleep [12].
Adenosine regulates sleep by inhibiting the excitability of the brain's arousal system via its receptors, activating sleep-promoting systems, and modulating homeostatic sleep (sleep pressure) and circadian rhythms. After falling asleep, adenosine is believed to prolong deep sleep or slow-wave sleep. It has also been found that the levels of adenosine may influence the functioning of the circadian clock and address the question of whether homeostatic sleep and the circadian clock may interact through adenosinergic signaling [13].
While it is still only hypothesized that adenosine is involved in the induction of sleep after prolonged wakefulness, a positron emission tomography study found that rapid eye movement sleep deprivation or sleep loss (which may be caused by sleep disturbances or sleep disorders) increases A1 receptor binding in the brain, providing evidence for A1AR upregulation in cortical and subcortical brain regions after prolonged wakefulness, which indicates its contribution to the homeostatic control of sleep [15]. Despite these findings, however, the precise mechanism of adenosine in regulating sleep and wakefulness remains unclear and warrants further research [15].
Adenosine and caffeine
Coffee, or more specifically caffeine, a major component of coffee, acts as an antagonist to adenosine receptors. In the brain, adenosine binding to its receptors normally causes drowsiness by slowing down nerve cell activity and allowing blood vessels to dilate, presumably to increase oxygen flow during sleep. However, to a nerve cell, caffeine structurally resembles adenosine, allowing it to bind to adenosine receptors.
Unlike adenosine, caffeine does not slow down cell activity; rather, it prevents the identification of adenosine by occupying its receptors, leading to an increase in nerve cell activity. As such, the effect of caffeine on sleep is usually opposite that of adenosine, which is both involved in sleep and necessary for sleep.
This is also why caffeine can cause constriction of brain blood vessels, countering adenosine's vasodilatory effect. This antagonistic interaction explains the stimulatory and arousal effects of caffeine, often used to combat drowsiness and even in headache medications to constrict blood vessels in the brain.
Adenosine and neuromodulation
Free adenosine in the brain is typically in the nanomolar range [16], but it can locally increase to millimolar levels during periods of low synaptic activity [17]. Acting primarily via A1R, adenosine is a presynaptic inhibitor of excitatory amino acid release and contributes to postsynaptic hyperpolarization [18].
As a potent neuromodulator, adenosine helps regulate synaptic activity, playing a well-established neuroprotective role [19,20]. Furthermore, adenosine's involvement in cognitive functions, such as learning and memory, is mediated by its interaction with A1 and A2A receptors, which influence processes related to memory formation and recall [21].
Adenosine and regulation of blood flow
Adenosine, released by astrocytes, stimulates vasodilation in endothelial cells via A2A receptors. This mechanism allows adenosine to regulate blood flow, facilitating increased local circulation. This response ensures adequate metabolic support during periods of intense synaptic stimulation [22].
Adenosine and neuroprotection
A2BR and A3R are expressed in the brain, although they are found at lower levels compared to A1R and A2AR [23] Furthermore, they exhibit lower affinity for adenosine, typically in the micromolar range [24]. As such, they are likely mediators of excessive adenosine signaling, such as in trauma, but further research is needed to fully understand their roles [25].
Unlike A1R and A2A, A2B and A3 receptors are mainly expressed in astrocytes. A2BR activation triggers interleukin-6 production, potentially contributing to the inflammatory response following brain trauma [26]. Additionally, the upregulation of A2BR in ischemic preconditioning suggests a role in neuroprotective mechanisms [27]. A3R activation appears to have a protective effect on astrocytes [28]. Modulation of adenosine receptors has been associated with neuroprotection in many model systems, such as hypoxia/ischemia [29,30], Parkinson’s disease [31,32], and epilepsy [33] among others.
Adenosine and pain modulation
Adenosine also plays a significant role in inflammatory and neuropathic pain, and one study reported that adenosine reduced neuropathic pain, hyperalgesia, and ischemic pain as well as morphine or ketamine [34]. Several clinical trials showed that preoperative adenosine reduced the amount of isoflurane required during surgery and postoperative opioid use [35,36].
Adenosine's impact on mood and anxiety
A2ARs are associated with depression-like symptoms, whereas A1R signaling elicits rapid antidepressant effects.
A2AR appears to be involved in anxiety disorders, as seen in studies showing increased depression-like behavior in transgenic rats overexpressing A2ARs [37]. Exposure to chronic unpredictable mild stress increases A2AR expression and triggers behavioral and synaptic alterations, which can be mitigated by caffeine, an antagonist (inhibitor) for A1 and A2A receptors [38]. In mice, inhibition of A2ARs by a selective antagonist enhances the activity of antidepressant medications [39].
In mice with switched on and off A1Rs, the activation of these receptors led to notable antidepressant effects [40]. Knockout mice lacking A1Rs exhibited increased depressive-like behavior that was resistant to the antidepressant effects of sleep deprivation, suggesting that the transient antidepressant effect observed after sleep deprivation is mediated by A1Rs [40].
Adenosine and neurodegenerative disorders
Growing evidence shows that adenosine and its receptors may be involved in the pathogenesis of Alzheimer’s disease. Adenosine and related metabolites are altered in Alzheimer’s disease patients [41,42]. Its receptors, A1R and A2AR, present differences in localization and density in brain regions affected by the disease [43,44]. A1R expression is downregulated in patients’ brains when analyzed postmortem, while A2AR expression is upregulated in the forebrain of the aged compared to young individuals [45]. Inhibition of A1R and A2AR seems to alleviate the neurotoxicity associated with β-amyloid accumulation and tau hyperphosphorylation and improves cognition and memory. However, further research is needed to fully understand the involvement of adenosine and its receptors in Alzheimer’s disease [46].
Targeting adenosine A2ARs may alleviate non-motor symptoms in Parkinson's disease including cognitive impairment, depression, and excessive daytime sleepiness. A2AR antagonists have shown efficacy in reversing cognitive deficits and depressive symptoms in rat models [47,48], and they may also improve motivation-related symptoms of depression [49]. Furthermore, istradefylline, an A2A antagonist, can enhance daytime wakefulness in Parkinson’s patients without affecting nocturnal sleep [50,51]. Overall, these findings suggest that A2AR antagonists have the potential to treat the neuropsychiatric components of Parkinson’s disease.
Oral bioavailability of adenosine
When it comes to the oral administration of adenosine, or more specifically its derivative adenosine-5'-triphosphate (ATP), studies have shown mixed results. While oral ATP administration has failed to increase plasma ATP levels, chronic supplementation has been demonstrated to enhance power, strength, lean body mass, and blood flow in trained athletes. This paradox raises questions about the bioavailability of adenosine and its effectiveness when administered orally.
In a study where subjects received varying doses of oral ATP for 28 days, only plasma uric acid levels showed a significant increase, particularly at the highest dose of 5000 mg. This suggests that while adenosine might not directly influence plasma ATP levels, its metabolites could have physiological effects
The benefits of adenosine supplementation
While research on adenosine supplements is still limited, some studies suggest that supplementing with adenosine may help regulate sleep patterns and promote better sleep.
-
Improved sleep quality: Adenosine supplementation may enhance the quality of sleep by promoting deeper and more restful sleep.
-
Reduced sleep latency: Adenosine supplements have been found to decrease the time it takes to fall asleep, helping individuals with insomnia or difficulty initiating sleep.
-
Enhanced sleep duration: Some studies indicate that adenosine supplementation can increase total sleep time, allowing individuals to obtain a sufficient amount of restorative sleep.
Considerations and precautions
-
Dosage: The optimal dosage of adenosine supplementation for sleep induction is still under investigation but very likely has a wide dose range.
-
Potential side effects: Adenosine supplementation is generally considered safe, but some individuals, especially those using high doses, may experience side effects such as headaches, dizziness, or gastrointestinal discomfort. If any adverse reactions occur, it is advisable to discontinue use and seek medical advice.
-
Interactions with medications: Adenosine supplements may interact with certain medications, including those for heart conditions or those with caffeine sensitivity, so be sure to ask your doctor first if you are currently on such medications.
- Intravenous adenosine is used in hospitals for cardiac issues including supraventricular tachycardia as it blocks AV nodal firing very briefly (a few seconds, max). Oral adenosine is much slower acting and has a much lower bioavailability overall, without any potential risk of cardiac complications.
Conclusion
In the brain, adenosine is a potent neuromodulator showing neuroprotective effects. With its receptors, adenosine participates in sleep regulation and pain modulation. Finally, alterations in adenosine concentration or receptor expression are associated with various diseases highlighting the significance of this molecule in the brain.
While further research is needed to fully elucidate the effects of adenosine supplements and supplementation, they may offer a promising avenue for those seeking to improve their sleep patterns.
Alternatively, you can also try Tro Zzz, our buccal troche formulated for sleep that has adenosine as one of its main ingredients, which will help you fall asleep, stay asleep, and wake up refreshed!
References
[1] Fredholm, B.B., IJzerman, A.P., Jacobson, K.A., Klotz, K.N. and Linden, J. (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacological Reviews, United States. 53, 527–52.
[4] Fredholm, B.B., IJzerman, A.P., Jacobson, K.A., Linden, J. and Müller, C.E. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and Classification of Adenosine Receptors—An Update. Pharmacological Reviews, 63, 1–34. https://doi.org/10.1124/pr.110.003285
[5] Rebola, N., Canas, P.M., Oliveira, C.R. and Cunha, R.A. (2005) Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience, 132, 893–903. https://doi.org/10.1016/j.neuroscience.2005.01.014
[6] Ciruela, F., Casadó, V., Rodrigues, R.J., Luján, R., Burgueño, J., Canals, M. et al. (2006) Presynaptic Control of Striatal Glutamatergic Neurotransmission by Adenosine A 1 –A 2A Receptor Heteromers. The Journal of Neuroscience, 26, 2080–7. https://doi.org/10.1523/JNEUROSCI.3574-05.2006
[7] Azdad, K., Gall, D., Woods, A.S., Ledent, C., Ferré, S. and Schiffmann, S.N. (2009) Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, England. 34, 972–86. https://doi.org/10.1038/npp.2008.144
[8] Ferre, S., Quiroz, C., Woods, A., Cunha, R., Popoli, P., Ciruela, F. et al. (2008) An Update on Adenosine A2A-Dopamine D2 Receptor Interactions: Implications for the Function of G Protein-Coupled Receptors. Current Pharmaceutical Design, 14, 1468–74. https://doi.org/10.2174/138161208784480108
[9] Rodrigues, R.J., Alfaro, T.M., Rebola, N., Oliveira, C.R. and Cunha, R.A. (2005) Co‐localization and functional interaction between adenosine A 2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. Journal of Neurochemistry, 92, 433–41. https://doi.org/10.1111/j.1471-4159.2004.02887.x
[10] Jamwal, S., Mittal, A., Kumar, P., Alhayani, D.M. and Al-Aboudi, A. (2019) Therapeutic Potential of Agonists and Antagonists of A1, A2a, A2b and A3 Adenosine Receptors. Current Pharmaceutical Design, 25, 2892–905. https://doi.org/10.2174/1381612825666190716112319
[11] Oishi, Y., Huang, Z.-L., Fredholm, B.B., Urade, Y. and Hayaishi, O. (2008) Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A 1 receptors and promotes non-rapid eye movement sleep. Proceedings of the National Academy of Sciences, 105, 19992–7. https://doi.org/10.1073/pnas.0810926105
[12] Morairty, S., Rainnie, D., McCarley, R. and Greene, R. (2004) Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience, 123, 451–7. https://doi.org/10.1016/j.neuroscience.2003.08.066
[13] Reichert, C.F., Deboer, T. and Landolt, H.P. (2022) Adenosine, caffeine, and sleep-wake regulation: state of the science and perspectives. J. Sleep Res., 31, 4, e13597. https://doi.org/10.1111/jsr.13597
[14] Elmenhorst, D., Meyer, P.T., Winz, O.H., Matusch, A., Ermert, J., Coenen, H.H., Basheer, R., Haas, H.L., Zilles, K. and Bauer, A. (2007) Sleep Deprivation Increases A
1 Adenosine Receptor Binding in the Human Brain: A Positron Emission Tomography Study. J. Neurosci. 27, 9, 2410-2415.
https://doi.org/10.1523/JNEUROSCI.5066-06.2007
[15] Huang, L., Zhu, W., Li, N., Zhang, B., Dai, W., Li, S. et al. (2024) Functions and mechanisms of adenosine and its receptors in sleep regulation. Sleep Medicine, 115, 210–7. https://doi.org/10.1016/j.sleep.2024.02.012
[16] Baraldi, P.G., Tabrizi, M.A., Gessi, S. and Borea, P.A. (2008) Adenosine Receptor Antagonists: Translating Medicinal Chemistry and Pharmacology into Clinical Utility. Chemical Reviews, 108, 238–63. https://doi.org/10.1021/cr0682195
[17] Ferré, S., Borycz, J., Goldberg, S.R., Hope, B.T., Morales, M., Lluis, C. et al. (2005) ROLE OF ADENOSINE IN THE CONTROL OF HOMOSYNAPTIC PLASTICITY IN STRIATAL EXCITATORY SYNAPSES. Journal of Integrative Neuroscience, 04, 445–64. https://doi.org/10.1142/S0219635205000987
[18] Cunha, R.A. (2008) Different cellular sources and different roles of adenosine: A1 receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A2A receptor-mediated facilitation of plasticity. Neurochemistry International, 52, 65–72. https://doi.org/10.1016/j.neuint.2007.06.026
[21] Dias, R.B., Rombo, D.M., Ribeiro, J.A., Henley, J.M. and Sebastião, A.M. (2013) Adenosine: setting the stage for plasticity. Trends in Neurosciences, 36, 248–57. https://doi.org/10.1016/j.tins.2012.12.003
[23] Dixon, A.K., Gubitz, A.K., Sirinathsinghji, D.J.S., Richardson, P.J. and Freeman, T.C. (1996) Tissue distribution of adenosine receptor mRNAs in the rat. British Journal of Pharmacology, 118, 1461–8. https://doi.org/10.1111/j.1476-5381.1996.tb15561.x
[26] Vazquez, J.F., Clement, H., Sommer, O., Schulz, E. and Van Calker, D. (2008) Local stimulation of the adenosine A 2B receptors induces an increased release of IL‐6 in mouse striatum: an in vivo microdialysis study. Journal of Neurochemistry, 105, 904–9. https://doi.org/10.1111/j.1471-4159.2007.05191.x
[27] Zhou, A.-M., Li, W.-B., Li, Q.-J., Liu, H.-Q., Feng, R.-F. and Zhao, H.-G. (2004) A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neuroscience Research, 48, 397–404. https://doi.org/10.1016/j.neures.2003.12.010
[28] Björklund, O., Shang, M., Tonazzini, I., Daré, E. and Fredholm, B.B. (2008) Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. European Journal of Pharmacology, 596, 6–13. https://doi.org/10.1016/j.ejphar.2008.08.002
[29] Rossi, D.J., Brady, J.D. and Mohr, C. (2007) Astrocyte metabolism and signaling during brain ischemia. Nature Neuroscience, 10, 1377–86. https://doi.org/10.1038/nn2004
[30] Stone, T.W. (2003) Purines and Neuroprotection. In: Alzheimer C, editor. Molecular and Cellular Biology of Neuroprotection in the CNS, Springer US, Boston, MA. p. 249–80. https://doi.org/10.1007/978-1-4615-0123-7_9
[31] Chen, J.-F., Sonsalla, P.K., Pedata, F., Melani, A., Domenici, M.R., Popoli, P. et al. (2007) Adenosine A2A receptors and brain injury: Broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Progress in Neurobiology, 83, 310–31. https://doi.org/10.1016/j.pneurobio.2007.09.002
[32] Kalda, A., Yu, L., Oztas, E. and Chen, J.-F. (2006) Novel neuroprotection by caffeine and adenosine A2A receptor antagonists in animal models of Parkinson’s disease. Journal of the Neurological Sciences, 248, 9–15. https://doi.org/10.1016/j.jns.2006.05.003
[34] Segerdahl, M., Ekblom, A. and Sollevi, A. (1994) The Influence of Adenosine, Ketamine, and Morphine on Experimentally Induced Ischemic Pain in Healthy Volunteers: Anesthesia & Analgesia, 79, 787???791. https://doi.org/10.1213/00000539-199410000-00029
[35] Segerdahl, M., Ekblom, A., Sandelin, K., Wickman, M. and Sollevi, A. (1995) Peroperative Adenosine Infusion Reduces the Requirements for Isoflurane and Postoperative Analgesics: Anesthesia & Analgesia, 80, 1145–9. https://doi.org/10.1097/00000539-199506000-00013
[36] Segerdahl, M., Persson, E., Ekblom, A. and Sollevi, A. (1996) Peroperative adenosine infusion reduces isoflurane concentrations during general anesthesia for shoulder surgery. Acta Anaesthesiologica Scandinavica, 40, 792–7. https://doi.org/10.1111/j.1399-6576.1996.tb04534.x
[37] Coelho, J.E., Alves, P., Canas, P.M., Valadas, J.S., Shmidt, T., Batalha, V.L. et al. (2014) Overexpression of Adenosine A2A Receptors in Rats: Effects on Depression, Locomotion, and Anxiety. Frontiers in Psychiatry, 5. https://doi.org/10.3389/fpsyt.2014.00067
[38] Kaster, M.P., Machado, N.J., Silva, H.B., Nunes, A., Ardais, A.P., Santana, M. et al. (2015) Caffeine acts through neuronal adenosine A 2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proceedings of the National Academy of Sciences, 112, 7833–8. https://doi.org/10.1073/pnas.1423088112
[39] Szopa, A., Bogatko, K., Serefko, A., Wyska, E., Wośko, S., Świąder, K. et al. (2019) Agomelatine and tianeptine antidepressant activity in mice behavioral despair tests is enhanced by DMPX, a selective adenosine A2A receptor antagonist, but not DPCPX, a selective adenosine A1 receptor antagonist. Pharmacological Reports, 71, 676–81. https://doi.org/10.1016/j.pharep.2019.03.007
[40] Serchov, T., Clement, H.-W., Schwarz, M.K., Iasevoli, F., Tosh, D.K., Idzko, M. et al. (2015) Increased Signaling via Adenosine A1 Receptors, Sleep Deprivation, Imipramine, and Ketamine Inhibit Depressive-like Behavior via Induction of Homer1a. Neuron, 87, 549–62. https://doi.org/10.1016/j.neuron.2015.07.010
[41] Alonso‐Andrés, P., Albasanz, J.L., Ferrer, I. and Martín, M. (2018) Purine‐related metabolites and their converting enzymes are altered in frontal, parietal and temporal cortex at early stages of Alzheimer’s disease pathology. Brain Pathology, 28, 933–46. https://doi.org/10.1111/bpa.12592
[42] Chang, C.-P., Wu, K.-C., Lin, C.-Y. and Chern, Y. (2021) Emerging roles of dysregulated adenosine homeostasis in brain disorders with a specific focus on neurodegenerative diseases. Journal of Biomedical Science, 28, 70. https://doi.org/10.1186/s12929-021-00766-y
[43] Albasanz, J.L., Perez, S., Barrachina, M., Ferrer, I. and Martín, M. (2008) RESEARCH ARTICLE: Up‐regulation of Adenosine Receptors in the Frontal Cortex in Alzheimer’s Disease. Brain Pathology, 18, 211–9. https://doi.org/10.1111/j.1750-3639.2007.00112.x
[44] Angulo, E., Casadó, V., Mallol, J., Canela, E.I., Viñals, F., Ferrer, I. et al. (2003) A 1 Adenosine Receptors Accumulate in Neurodegenerative Structures in Alzheimer’s Disease and Mediate Both Amyloid Precursor Protein Processing and Tau Phosphorylation and Translocation. Brain Pathology, 13, 440–51. https://doi.org/10.1111/j.1750-3639.2003.tb00475.x
[45] Temido-Ferreira, M., Ferreira, D.G., Batalha, V.L., Marques-Morgado, I., Coelho, J.E., Pereira, P. et al. (2020) Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Molecular Psychiatry, 25, 1876–900. https://doi.org/10.1038/s41380-018-0110-9
[46] Trinh, P.N.H., Baltos, J.-A., Hellyer, S.D., May, L.T. and Gregory, K.J. (2022) Adenosine receptor signalling in Alzheimer’s disease. Purinergic Signalling, 18, 359–81. https://doi.org/10.1007/s11302-022-09883-1
[47] Prediger, R.D.S., Da Cunha, C. and Takahashi, R.N. (2005) Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behavioural Pharmacology, 16, 209–18. https://doi.org/10.1097/01.fbp.0000166825.62130.9a
[48] Kadowaki Horita, T., Kobayashi, M., Mori, A., Jenner, P. and Kanda, T. (2013) Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology, 230, 345–52. https://doi.org/10.1007/s00213-013-3158-x
[49] Chamberlain, S.E.L., Sadowski, J.H.L.P., Teles-Grilo Ruivo, L.M., Atherton, L.A. and Mellor, J.R. (2013) Long-Term Depression of Synaptic Kainate Receptors Reduces Excitability by Relieving Inhibition of the Slow Afterhyperpolarization. The Journal of Neuroscience, 33, 9536–45. https://doi.org/10.1523/JNEUROSCI.0034-13.2013
[50] Suzuki, K., Miyamoto, M., Miyamoto, T., Uchiyama, T., Watanabe, Y., Suzuki, S. et al. (2017) Istradefylline improves daytime sleepiness in patients with Parkinson’s disease: An open-label, 3-month study. Journal of the Neurological Sciences, 380, 230–3. https://doi.org/10.1016/j.jns.2017.07.045
[51] Matsuura, K., Kajikawa, H., Tabei, K., Satoh, M., Kida, H., Nakamura, N. et al. (2018) The effectiveness of istradefylline for the treatment of gait deficits and sleepiness in patients with Parkinson’s disease. Neuroscience Letters, 662, 158–61. https://doi.org/10.1016/j.neulet.2017.10.018





Comments (0)
There are no comments for this article. Be the first one to leave a message!